Microbiology Culture Specimens
Microbiology Culture Specimens for culture should be obtained prior to the administration of antimicrobial agents.
- If obtaining pre-antimicrobial specimens is not possible, inform the laboratory about the therapeutic agent(s) used. This information should be considered before issuing the laboratory report.
- Collect material from the appropriate site with a high likelihood of isolating suspected organisms.
- In cases where patient participation is required for sample collection (e.g., sputum or urine), ensure proper and clear instructions are given.
- Adequate specimen quantity must be collected to allow comprehensive examination.
- Place specimens in sterile containers to maintain their integrity.
- Some specimens are directly collected in culture media; in such cases, contact the laboratory for specific instructions.
- Accurate labelling of specimens is essential, including patient’s name, test type, collection date, and site.
- Record relevant clinical information on the request form.
- Note any special procedures required due to unique conditions, circumstances, or situations on the request form.
- Collect specimens during regular working hours, unless in emergencies, to ensure the availability of qualified microbiologists for supervision.
- The selection of appropriate specimens for isolating viral, chlamydial, or rickettsial agents depends on the nature of the illness.
- Obtain materials as early as possible during the acute phase of the disease, as these agents tend to diminish quickly after symptom onset.
- When collecting vesicle fluid, use a syringe or capillary pipette and dilute it immediately with an equal volume of skimmed milk or tissue culture medium.
- Freeze and store all viral culture specimens at -70°C until the culture initiation.
THROAT AND NASAL SWABS
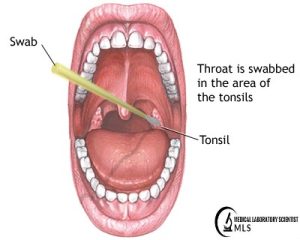
- Take throat swab cultures under direct vision with proper lighting.
- Choose areas with exudation, membrane formation, inflammation, or, if not visible, tonsillar crypts.
- Nasopharyngeal swabs are best taken by the treating physician or surgeon.
- For recovering viral agents, collect washings after gargling with nutrient broth.
NASAL SPECIMEN For Mycobacterium leprae
- For obtaining a nasal specimen for M. leprae, follow these steps: Nasal Swab
- Seat the patient with their head tilted backward facing the light.
- Gently insert and rotate the swab in one nasal cavity against the upper part of the nasal septum.
- Create 2-3 evenly spread smears.
- Air-dry the slides, wrap them, and send to the laboratory.
Nasal Washings & Nasal Blow
- Seat the patient. Place a few drops of sterile saline in their nose.
- After 3 minutes, instruct the patient to blow their nose forcefully on plastic or cellophane sheet. (Give this sheet to the patient for use at home the following morning, immediately upon waking and before washing, to bring directly to the laboratory).
- Transfer mucus from the washing to a slide using a clean wooden stick to make a thin smear.
- Air-dry the slide and send it to the testing area.
SPUTUM SPECIMEN
- Collect an early morning sputum specimen.
- Provide the patient with a clean, dry, wide-necked, leak-proof container.
- Instruct the patient to cough deeply to produce sputum.
- For Tuberculosis culture, collect three fresh, early morning specimens (5-10 ml each) and refrigerate them. If the amount is insufficient, advise the patient to collect sputum over 24 hours or until reaching 50 ml.
- For patients unable to provide sputum, Tuberculosis can be recovered from gastric contents via gastric aspiration performed indoors.
- Collect gastric washings early in the morning in a fasting state. Neutralize them immediately with N/10 NaOH.

FAECAL SPECIMEN
- Rectal swabs aid in identifying the cause of acute bacterial diarrhoea when stool collection is challenging.
- Directly pass faeces into a clean, waxed cardboard container with a tight cover.
- Ensure no residual soap, detergent, or disinfectant is present in the container, as these can affect the specimen.
- Transfer faeces to another clean container. Include pus, blood, mucus, or formed elements passed with stool, covering the first, last, and middle portions.
- Add approximately 1 ml of specimen to 10 ml sterile alkaline peptone water in suspected cholera cases.
- In case of suspected viral infection, extract faeces with sterile buffered saline. Mix 1 ml of faeces with 9 ml sterile buffered saline, allow sedimentation, transfer the supernatant to a sterile container, freeze it below -40°C until processing. (Collect paired sera at the same time and after 2-3 weeks).

URINE SPECIMEN
Urine specimens are often self-collected by patients. Proper instructions are vital for correct sample collection. An uncontaminated mid-stream urine (MSU) sample is ideal, and the following methods ensure its collection:
Females
- Thoroughly wash the genital area with soap and water. Hold the labia apart with two fingers, rinsing the area from front to back with soap and tap water.
- Begin urinating so that the stream does not touch the skin. After a brief interval, position a sterile container beneath the urine stream.
- Remove the container from the as soon as the necessary amount of urine has been collected.
Males
- Wash the genital area thoroughly with soap and water.
- Begin urinating and place a sterile container under the stream after a few moments. Collect the required amount and remove the container.
- Securely cap the container.

Infants, Uncooperative, and Debilitated Patients
- Attach plastic bags after thorough washing of the genital area.
- Monitor bags for immediate removal after urine passage.
- If urine isn’t passed within 30 minutes, remove the collecting bag.
- Re-scrub the patient and attach a new collection device.
Urine Collection for Mycobacterium tuberculosis
- Three consecutive early morning specimens (>90 ml each) collected in a sterile container are preferable to a 24-hour collection.
- Use boric acid (1.6%) as a preservative in exceptional cases, such as 24-hour urine collection, when daily reporting for sampling is challenging.
- Suprapubic aspiration in a medical setting is preferred for catheterized patients.













































[…] Microbiology Culture Specimens: Types, Collection, Transport […]