MANTOUX TEST
Mantoux test is used as a screening tool for tuberculosis (TB) which is caused by a bacterium Mycobacterium tuberculosis. M. tuberculosis is an aerobic bacteria discovered by Dr. Robert Koch in 1882. Tuberculosis is one of the poverty-related diseases (PRDs) along with AIDS and malaria. The Mantoux test was developed by a French scientist Charles Mantoux in 1907 and is the most frequently performed procedure in epidemiological surveys and assessment of tuberculosis treatment with anti-TB drugs. The antigen used in the test is called PPD (purified protein derivative) which is an extract of Mycobacterium tuberculosis.
PRINCIPLE
-
- PPD (purified protein derivative) is subcutaneously injected to the patient and individual’s sensitivity to tuberculin protein is assessed. If the patient already had a previous exposure to M. tuberculosis, a hypersensitivity reaction takes place.
- The antigen produces induration (thickening) at the site of injection. The diameter of the induration is measured to evaluate the test and is directly proportional to the level of sensitization. American Thoracic Society (ATS) has recommended 5 Tuberculin Units (0.1 ml) as a standard regimen for the Mantoux Test.
- In highly endemic areas, this standard dose could be reduced as per the guidelines.
REQUIREMENTS
-
- Graduated syringe (of 1 ml) with a short bevel needle (26 G)
- Tuberculin PPD (Mantoux solution)
- Spirit swab
PROCEDURE:
-
- Bring the tuberculin PPD to room temperature
- Draw up just over 0.1ml of tuberculin protein into a graduated syringe.
- Remove air and excess tuberculin to leave exactly 0.1ml of tuberculin.
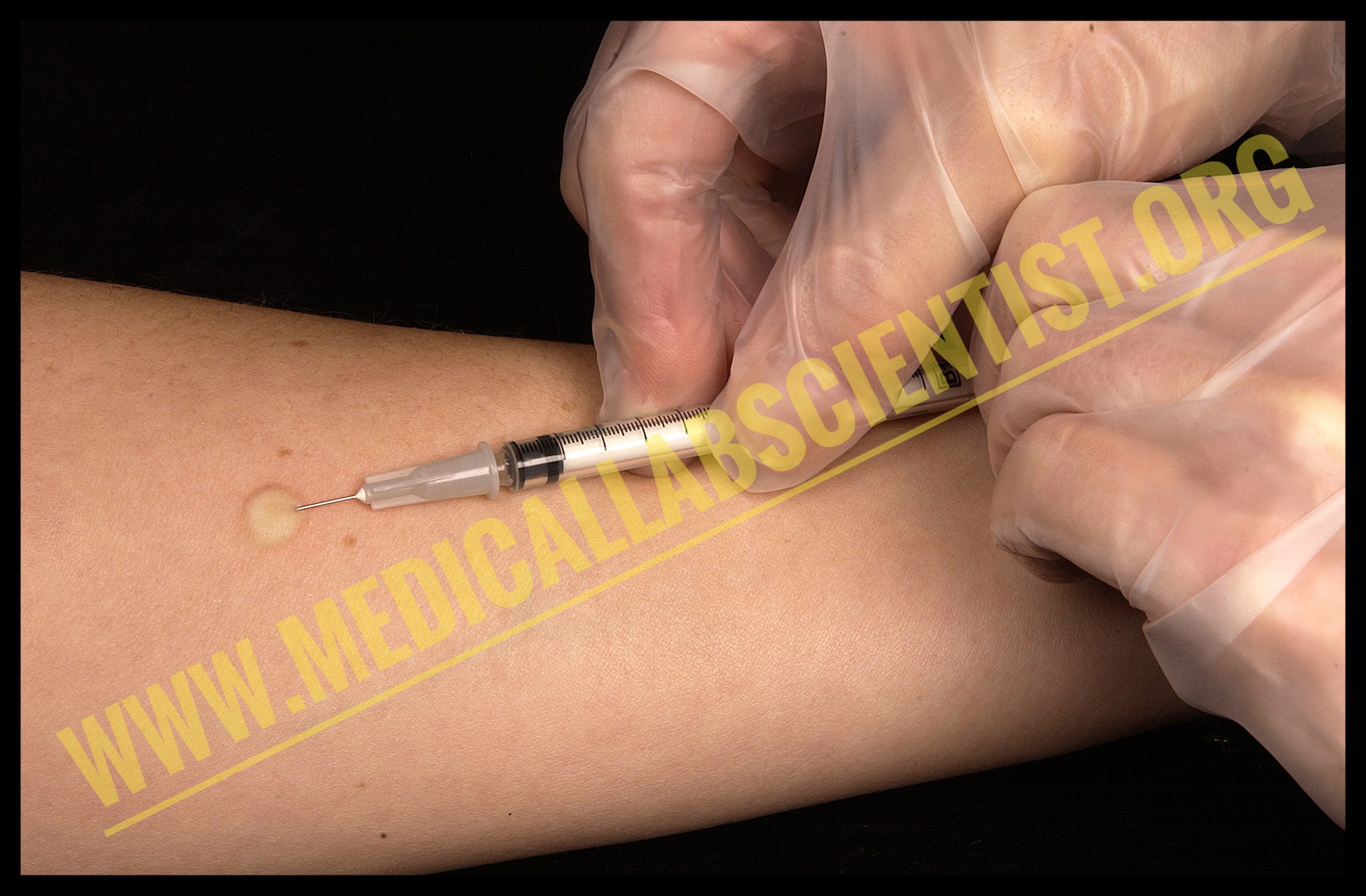
- The ideal site is dorsal surface of the forearm (usually left) about 4 cm below the elbow joint.
- Clean the site with spirit swab and allow to dry.
- Insert the needle parallel with the skin surface, advance the bevel 3-5 mm and inject the PPD.
- Remove the needle without pressing the injection site.
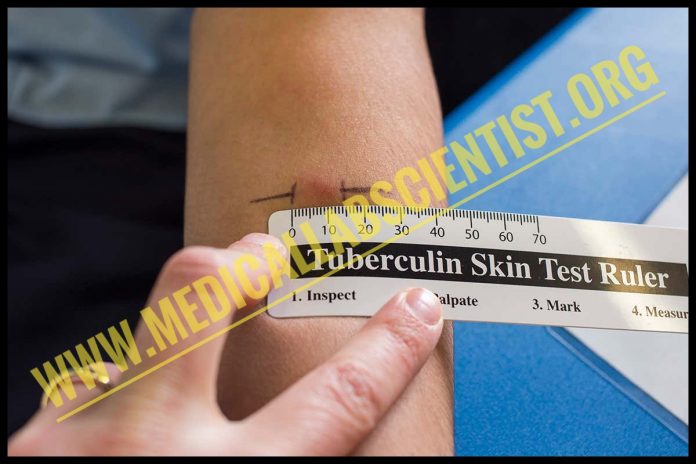
- Wait for 48-72 hours and observe the induration. The area of erythema is not included.
RESULTS:
-
- Induration of 15 mm or more is considered positive in a person with no known risk factors.
- Induration of 10 mm or more indicates a positive reaction in high risk factor individuals while induration of less than 5 mm indicates a negative reaction.
- Induration of 5-9 mm is considered positive in individuals with immune deficiencies, e.g. AIDS patients and organ transplant patients.
- In normal individuals, 5-9 mm reaction is doubtful and indicative of re-testing.
INTERPRETATION OF MANTOUX TEST:
-
- A positive Mantoux test indicates a previous exposure to M. tuberculosis but not necessarily an active disease.
- The Mantoux test causes a booster effect to produce a positive result in an individual who has been already exposed previously.
- The Mantoux test usually becomes positive 4-6 weeks after infection.
- If a papule does not appear after the injection, the test should be repeated on other arm as the solution has been injected too deeply.
- False negative results are seen if there is bacterial contamination of the tuberculin solution, corticosteroid therapy, viral infections (HIV, influenza, EBV) and poor nutrition.
- False positive results are seen in individuals vaccinated with BCG (reactions are 5-10 mm diameter).
- If the induration is 15 mm or more in BCG vaccinated individual, it should be considered positive.